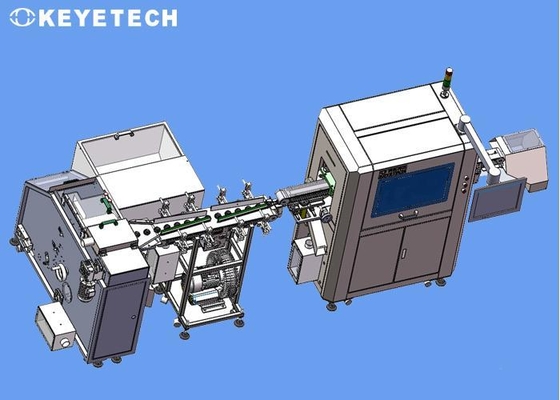
360 Degree Cap Vision Inspection System For 20mm~38mm Bottle Closures
Company Profile
Anhui Keye Intelligent Technology Co., Ltd. is a Chinese national high-tech enterprise specializing in the application of AI technology in visual inspection industry. As a leading manufacturer&Designer, It can provide the most advanced level AI visual inspection equipment and the customized AI system solutions of visual imaging, computing power, algorithm, and automatic control software for various sub-industries.

The company was established in 2011, it has a high-level R&D team from top domestic optical research institutes and a core doctoral team from the USTC, covering the entire technology chain of optics, electrics, mechanics, algorithm and software. Our products can be applied in beverage, pharmacy, dairy, wine, textile, new energy, agriculture and other packaging industries.
Taking 9~12mm medicine bottle caps as an example, our company's design is equipped with high precision industrial cameras to perform full range of defect detection on the concave surface, trademark surface and side of the bottle cap. The normal detection speed is 1500 pieces/min; The front end of the inspection machine can be equipped with a set of sorting and conveying equipment to transport bottle cap products into the inspection equipment in a regular and orderly manner.

The inspection equipment is divided into three major inspection system modules. The product is adsorbed and transported by the vacuum adsorption method for defect detection on the trademark surface, and the unqualified products can be automatically rejected; the equipment can realize 7*24 hours of operation. The software will open the sample comparison standard, and the production personnel on the customer site can control the comparison accuracy and select the accuracy standard most suitable for their own production conditions, thereby controlling the defect rate.
The standards are as follows:
| Model |
Number of camera |
Inspection scope |
Inspection content |
Detection precision |
Accuracy |
Capacity |
Remark |
| KVIS-CC06 |
4sets |
Outside surface |
Black spot≥0.2mm |
99.8% |
99% |
500pcs/min |
|
| 0.5mm<Flash, injection incomplete≤1mm |
99% |
| 1set |
Concave surface |
0.5m<Injection incomplete≤1mm |
99.8% |
| Black spot≥0.2mm |
99.8% |
| 2mm<Transformation |
99% |
| 1set |
Trademark surface |
Black spot≥0.2mm |
99.8% |
| Note: The system equipped with 2 million pixel-level industrial cameras. |
The counting function can be realized after the finished product is tested, and the counting accuracy rate is 100%.
During use, the testing equipment needs to be maintained and cleaned regularly (such as conveyor belt cleaning, camera cleaning, etc.). The testing equipment itself will not cause secondary pollution sources (such as debris, fine foam, etc.) to the tested samples.
Software function:
Enabling and disabling the system detection function
Adjustment of detection index parameters and accuracy.
Camera screen calibration function
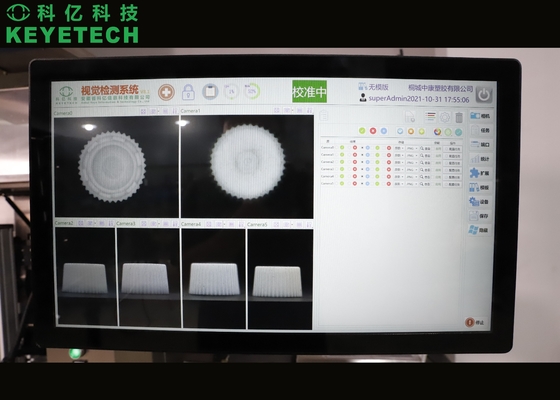
Real-time display system production information (qualification rate, total output, speed, etc.), browse historical production information, export and store functions. Template sampling and storage, sample detection information storage and recall.
Permission management of login accounts (such as general operation users disable parameter adjustment permissions)
Operation and adjustment of the mechanical part of the system
(Such as the transmission device is opened, the air valve injection time is adjusted, and the alarm signal is adjusted)
Reference pic of the sample

The rapid development of the national society has put forward higher requirements for the quality of pharmaceutical production, and the downstream pharmaceutical quality and safety requirements have increased, promoting the upgrading of the pharmaceutical packaging industry, which is conducive to improving the level of pharmaceutical quality and safety, thereby promoting orderly competition and competition in the upstream pharmaceutical packaging industry.
Cooperation Partners
KEYETECH has always been committed to the application of artificial intelligence in the field of vision technology, replacing human eyes and brain decision with machine vision and AI reasoning calculations, and integrating the quality detection and sorting to industrial products in traditional manufacturing to make the production more digitization , Intelligence, visualization, and persistence. Also greatly improves the efficiency and accuracy of industrial detection, and enhances the intelligent level of the manufacturing industry.


 Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!  Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!